|
Physicians and Surgeons have believed in the regenerative properties of placental derived allografts since the early 1900’s. Long before they knew the healing science of biomaterials derived from placental tissues, surgeons were amazed at how their patients healed faster, with less pain, less scar tissue and fewer infections, when using placental derived allografts in their care of their patients.
Human amniotic membrane is comprised of the innermost layer of the placenta and lines the amniotic cavity. The membrane is composed of multiple layers, including a single layer of epithelial cells, a basement membrane and an avascular connective tissue matrix. The tissues of the placenta present a very complex interrelationship of materials that possess numerous physiologic characteristics that can in turn change in importance with the appropriate stage of gestation. During pregnancy, the placenta permits the passage of nutrients, metabolites and metabolic gases, and provides physical and immunological protection to the developing fetus. In addition, it produces a variety of steroids and important metabolic hormones.
Amniotic membrane is a unique material and its composition contains collagen types I, III, IV, V, and VII. It is composed of structural extracellular matrix (ECM) that also contains specialized proteins fibronectin, laminins, proteoglycans and glycosaminoglycans. In addition, amniotic membrane contains essential active healing growth factors such as epidermal growth factor (EGF), transforming growth factor beta (TGF-b), fibroblast growth factor (FGF), and platelet derived growth factor (PDGF). Amniotic tissues have shown little to no HLA-A, B, C antigens and β2 microglobulin.
AmnioFix® is a composite amniotic tissue membrane, minimally manipulated to protect the collagen matrix and its natural properties. AmnioFix® reduces scar tissue formation, modulates inflammation in the surgical site, enhances healing, and acts as a barrier.
Human amniotic membrane comprises the innermost layer of the placenta and lines the amniotic cavity. AmnioFix® is processed through the proprietary PURION® Process that combines cleaning, dehydration and sterilization, and it may be stored at ambient conditions for up to five years. The proprietary PURION® Process protects the delicate scaffold during processing, leaving an intact collagen matrix. The result is a durable graft with natural barrier properties to optimize surgical performance and ease of use. AmnioFix® is available in sheet/membrane, particulate, and wrap configurations for use in surgical, soft tissue, tendon, and nerve applications.
Surgical applications are limitless, as there isn't a surgery that can’t benefit from the regenerative properties of human Amnion/Chorion allografts.
Patient wound healing is significantly enhanced, wounds have less scar tissue, patients experience less pain and the risk of infection is reduced.
Surgical case history:
A 52 year-old female with a one-and-a-half year history of chronic Hallux limitus of right foot. The patient failed to respond to conservative care including: NSAIDs, orthotics, activity modification, a change in shoes and cortisone injections with two prior physicians. An X-ray exam revealed stage 3 Hallux Limitus involving damage to both proximal and distal joint segments. The surgical options were reviewed and the patient revealed a desire to continue tennis and ballroom dancing, so fusion was not an option. The patient was scheduled first for MPJ arthroplasty with a total implant. The surgical technique was augmented with the application of AmnioFix® allograft over the 1st MPJ prior to capsular closure and additional AmnioFix® allograft over the capsule prior to soft tissue closure. The patient reported no need for narcotic pain medication post op, she was comfortable with oral Tylenol and ice as scheduled. The patient was able to begin range of motion immediately post op and upon removal of steri-strips, she returned to soft shoe gear at fourteen days post op. The patient continued ROM home exercises and returned to tennis by eight weeks.
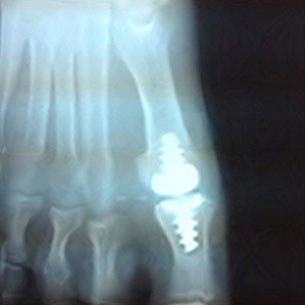
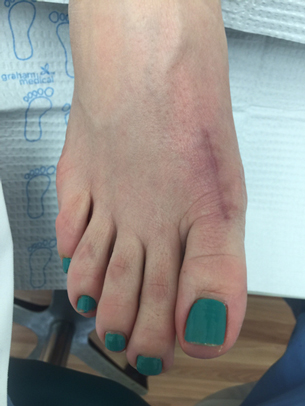
AmnioFix® allograft significantly reduced her pain and scar tissue both at the capsular level and subcutaneously. Most surgeons who do foot and ankle surgery and especially implant arthroplasty struggle with swelling and post op joint stiffness. The results of this case and numerous others revealed significant reduction in post op pain, joint stiffness and much earlier return to normal shoe gear and activities.
AmnioFix® is available in numerous sizes to minimize waste and optimize cost. Also, as the product is dehydrated, it is easily handled and can stored on the shelf at your institution for up to five years.
###
-
Niknejad H; Peirovi H; Jorjani M; Ahmadiani A; Ghanavi J; Seifalian AM "Properties of the amniotic membrane for potential use in tissue engineering." Eur Cell Mater. (15). 01-JAN-2008. pp 88 - 99.
-
Rahman I; Said DG; Maharajan VS; Dua HS "Amniotic membrane in ophthalmology: indications and limitations." Eye. (23)10. 2009. pp 1954–1961.
-
Baradaran-Rafii A; Aghayan H; Arjmand B; and Javadi M. "Amniotic Membrane Transplantation." Iran J Ophthalmic Res. (2)1. 2007. pp 58-75.
-
John, T. "Human amniotic membrane transplantation: Past, present, and future.." Ophthal Clin N Am. (16). 2003. pp 43-65.
-
Adly OA; Moghazy AM; Abbas AH; Ellabban AM; Ali OS; Mohamed BA "Assessment of amniotic and polyurethane membrane dressings in the treatment of burns." Burns - 01-AUG-2010; 36(5): 703-10.
-
Huiren Tao & Hongbin Fan "Implantation of amniotic membrane to reduce postlaminectomy epidural adhesions." Eur Spine J - 01-AUG-2009; 18(8): 1202-12. (). 2009.
-
Arora R; Mehta D; Jain V "Amniotic membrane transplantation in acute chemical burns." Eye (Lond). (19)3. 01-MAR-2005. pp 273-8.
-
Kay H; Nelson D; Wang Y. “The Placenta: From Development to Disease.” Wiley-Blackwell. 2011.
|
